2001
Basel, Switzerland
Modeling drug induced changes in biomarkers without using drug concentrations: Introducing the K-PD model
Philippe Jacqmin1, Ronald Gieschke2,4, Paul Jordan2,4, Jean-Louis Steimer2,4, Timothy Goggin2,3, Goonaseelan (Colin) Pillai2,3, Eric Snoeck1, Pascal Girard1.
1Pharsight Corporation, Mountain View, California 94040, USA
Biomarkers that trace physiologic or biochemical variations related to drug action are of great interest in early drug development. PK-PD models, as indirect response models (IRMs), improve the use of these biomarkers e.g. for performing predictive simulations. Generally, effects described by IRMs require the concentration-time profile of the drug as input. However, longitudinal analysis of biomarker data often reveals that they contain strong kinetic components related to the physiological or biochemical processes which may be rate limiting in the PK/PD system as a whole. We postulate that, if the biomarker data are rich enough, a PD model can be derived without drug concentrations, using the following adapted IRM (see Fig.)
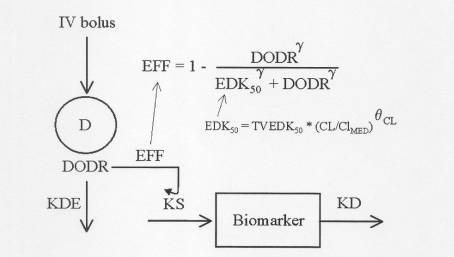
The model presented here shows bolus input of a drug and inhibition of the synthesis rate (KS;time-1) of the biomarker. This model can easily be extended to other types of IRMs as well as to different drug input functions. The inhibition is modeled with a sigmoid Emax model in which the independent variable is a virtual DOse Driving Rate (DODR; amount.time-1) defined as the product of the output rate (KDE; time-1) from a virtual intermediary compartment (into which the drug is administered) and the amount in this compartment. It takes care of the equilibration kinetics between the drug administration rate and the drug effect and is analogous to the Keo in a link model. EDK50 represents the DODR that leads to 50% inhibition of KS. The K character in EDK50 indicates that, besides the PD variability, the apparent potency of the drug is also influenced by dosage form and PK variability. E.g. it can be estimated whether drug clearance CL or a covariate of CL (by propagation), has a relevant effect on EDK50. The model is named the kinetic and pharmacodynamic (K-PD) model.
Using population simulations and analyses, the performance of the K-PD model in different settings is compared with the classical approach using plasma concentrations and turns out to give an excellent performance in terms of model fits, robustness and computational time. The K-PD model has already been applied to various drugs and drug actions (see Goggin et al. PAGE 2001, Gieschke et al. PAGE 2001, Pillai et al. PAGE 2001). An analytical solution for the K-PD model, comprising also steady-state, is proposed.