Population Pharmacokinetics And Therapeutic Response Of Artemether Following Treatment Of Coartem In Malaria Patients
Farkad Ezzet
Novartis Pharma Ag., K-490.3.32, CH-4002 Basel, Switzerland.
Two hundred and sixty patients were enrolled into a randomised, double-blind, parallel group, dose-finding trial of a new antimalarial combination in the treatment of acute, uncomplicated P. falciparum malaria in Thailand. Coartem (artemether and benflumetol) was given orally, either as: A, 4 x 4 tablets over 48 hours; B, 4 x 2 tablets over 48 hours or C, 3 x 4 tablets over 24 hours.
Using a population-based approach combining full profiles (42 patients) and sparse data (218 patients), the pharmacokinetic (PK) and pharmacodynamic (PD) properties of artemether and its active metabolite, dihydroartemisinin were described.
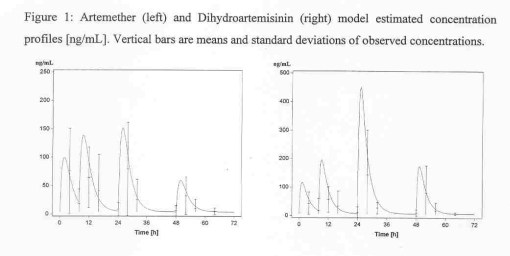
Overall, artemether was rapidly absorbed (half-life 1.9 h) and converted to dihydroartemisinin at a similar rate. Peak concentrations of artemether and dihydroartemisinin were reached at 1.8 h and 1.2 h, and the terminal elimination half-lives were 0.84 and 0.43 h, respectively. The inter-patient variability in Ka, CL and in F1 - F4 (bioavailability fractions of doses 1 to 4) was large. This may be explained by inter-individual variability in CYP3A activity as this is probably the main route of metabolism of artemether. The correlation coefficients between artemether and dihydroartemisinin levels based on AUC within each dosing interval were 0.42, 0.47, 0.61 and 0.65. The corresponding artemether: dihydroartemisinin ratios were 1.9, 1.1, 0.9, and 0.8, Figure 1, suggesting that the metabolism of artemether may also increase with time.
Higher artemether or dihydroartemisinin AUC(0-∞) was found to significantly decrease parasite clearance time, but to have a negligible effect on prevention of recrudescence.