



Next: 35
Carbamazepine Population Pharmacokinetics
Up: PAGE '95: ABSTRACT LIST
Previous: 33
An Industrial Perspective:
M. Sandström(1) ,
A. Freijs(1) , R. Larsson(2) , P. Nygren(3) , J. Bergh(3) , M-L. Fjällskog(3) ,
M.O. Karlsson(1) ,
Department of Biopharmaceutics and Pharmacokinetics, Uppsala
University, Sweden(1)
Division of Clinical Pharmacology(2) and Department of Oncology(3) ,
University Hospital,
Uppsala University, Sweden
Purpose
The aim of this study was to investigate the covariance between
the pharmacokinetics of the three components of the FEC regimen,
epirubicin (EPI),5-fluorouracil (5FU) and the cyclophosphamide
(CP) metabolite 4-hydroxycyclophosphamide (4-OHCP), in breast
cancer patients.
Patients and methods
Data from 21 women were collected over a total of 35 cycles. 5FU
(300-600mg/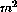 ) and CP (300- 600mg/
) and CP (300- 600mg/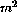 ) were given as bolus
injections, whereas EPI (15-60mg/
) were given as bolus
injections, whereas EPI (15-60mg/ 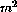 ) was given either as a
bolus injection or as an infusion. The pharmacokinetics of the
component drugs were monitored using a limited sampling scheme.
Population pharmacokinetic models for each of the three drugs
were developed using the program NONMEM.
) was given either as a
bolus injection or as an infusion. The pharmacokinetics of the
component drugs were monitored using a limited sampling scheme.
Population pharmacokinetic models for each of the three drugs
were developed using the program NONMEM.
Results
The data for 5FU was best described by a one-compartment model
with nonlinear elimination, where the maximal rate of elimination
(Vmax) and the concentration at which the elimination was
half-maximal (Km) were 105mg/L/h and 27mg/L, respectively. EPI
concentration-time profiles showed a triexponential decline with
an average terminal half-life of 24h and a clearance of 59L/h.
The elimination of 4-OHCP was monoexponential with an average
half-life of 7h. The interindividual coefficient of variation in
apparent clearance was 30%, 22%, and 41% for 5FU, EPI and
4-OHCP, respectively. The corresponding values for intrapatient
course-to-course variability in clearance were 11%, 8% and
27%. No significant correlation in any of the pharmacokinetic
parameters between the drugs was found.
Conclusion
Dose escalation to maximal tolerated dose, individualisation
using therapeutic drug monitoring or attempts to find
concentration-response relationships may be successful but
requires that the exposure of all three drugs are considered
simultaneously.




Next: 35
Carbamazepine Population Pharmacokinetics
Up: PAGE '95: ABSTRACT LIST
Previous: 33
An Industrial Perspective:
harnisch@pollux.zedat.fu-berlin.de
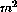 ) and CP (300- 600mg/
) and CP (300- 600mg/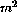 ) were given as bolus
injections, whereas EPI (15-60mg/
) were given as bolus
injections, whereas EPI (15-60mg/ 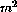 ) was given either as a
bolus injection or as an infusion. The pharmacokinetics of the
component drugs were monitored using a limited sampling scheme.
Population pharmacokinetic models for each of the three drugs
were developed using the program NONMEM.
) was given either as a
bolus injection or as an infusion. The pharmacokinetics of the
component drugs were monitored using a limited sampling scheme.
Population pharmacokinetic models for each of the three drugs
were developed using the program NONMEM.