B. Tranchand, S. Urien, C. J. Ardiet
Centre Leon Berard, Lyon Cedex 08, France
Introduction
The alkylating agent melphalan has been used for 30 years in a variety of malignant tumors. Previous pharmacokinetic studies of intravenous melphalan have demonstrated large interindividual variations in the pharmacokinetic parameters.
Aim of the study
The present study was conducted in order to estimate population pharmacokinetic parameters of melphalan, and to look at a relationship between the pharmacokinetic parameters and the following covariates: age, sex, weight, body, surface area, creatinine clearance, previous treatments.
Methods
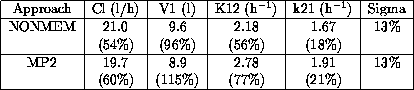
Data were obtained from 41 patients who received a single dose of intravenous melphalan. The sampling schedule was as follows: prior to the administration of melphalan then 5, 10, 15, 30, 45, 60, 90, 120, 180 min. Melphalan was assayed by HPLC. Population analysis was performed using NONMEM and MP2. A biexponential pharmacokinetic model was chosen to describe the plasma level data, and the model was parametrized in terms of: Cl, V1, k12, k21. The effect of covariates on pharmacokinetic parameters was investigated.
Result
Results obtained from the two approaches NONMEM and MP2 were similar (parameter estimates, coefficient of variation):

The main significant relationships were found between weight and Cl and V1. Sex seems to have a significant effect on Cl. Validation of the model is currently being performed using further kinetics.