



Next: 31
Performance Evaluation of
Up: PAGE '95: ABSTRACT LIST
Previous: 29
Modeling Interoccasion Variability
L.S. Murray(1) ,
D.I. Jodrell(2) . J.G. Morrison(2) , A.W. Kelman(1) , D.J. Kerr(2) , B.Whiting(1) & S.B. Kaye(2)
Departments of Medicine & Therapeutics(1) and Medical Oncology(2)
University of Glasgow
Introduction
In clinical practice, the dosage used in cytotoxic drug therapy
is usually empirical, based on body surface area, and not on the
results of formal pharmacokinetic (PK)/pharmacodynamic (PD)
studies. It is now accepted that the pharmacodynamics of many
drugs are better related to pharmacokinetic parameters such as
the area under the plasma concentration time curve (AUC) or
concentration at steady state, than to absolute dose (1). Current
practice therefore fails to maximise the chance of achieving the
therapeutic objectives of tumor reduction and increased survival
whilst minimizing toxity. Development of a dosage strategy based
on PK/PD studies requires firstly the identification of a target
range for the PK parameter which is associated with the required
PD response, and secondly the ability to maintain the PK
parameter within target range by dosage adjustment within an
individual. The aim of this study was to determine whether it was
possible, with the anti-cancer drug epirubicin, which is given at
21 day intervals, to determine the plasma concentrations which
will be achieved in after each course of treatment.
Patients and Methods
As part of the routine treatment for breast cancer 41 patients
received 100mg/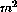 epirubicin, and 25mg prednisolone twice
daily for 5 days. 35 of these patients were randomised to receive
quinidine (250mg), as a modulator of resistance to epirubicin, or
placebo for 4 days prior to and 2 days after chemotherapy as part
of an on-going trial. Unless there was disease progression or
undue toxicity patients received 8 cycles of chemotherapy.
Epirubicin was given by a short intravenous infusion over 5-10
mins on the first cycle blood samples were taken at 0, 5, 10, 20,
40, 60 and 90 minutes, and 2, 4, 8, 12, 20 and 24 hours after the
end of the infusion. These were centrifuged and the plasma frozen
until analysed by HPLC. On subsequent cycles samples were taken
at 10mins, 1hr and 24hrs after the end of the short infusion.
Clinical information such as height, weight, age and
pre-treatment biochemistry was also recorded for each of the
patients. A population PK model was developed using the computer
program NONMEM (2). The population parameter estimates were then
used in a Bayesian Algorithm to produce posterior PK parameter
estimates for the individual, which were in turn used to predict
the epirubicin concentrations in that individual following the
next dose.
epirubicin, and 25mg prednisolone twice
daily for 5 days. 35 of these patients were randomised to receive
quinidine (250mg), as a modulator of resistance to epirubicin, or
placebo for 4 days prior to and 2 days after chemotherapy as part
of an on-going trial. Unless there was disease progression or
undue toxicity patients received 8 cycles of chemotherapy.
Epirubicin was given by a short intravenous infusion over 5-10
mins on the first cycle blood samples were taken at 0, 5, 10, 20,
40, 60 and 90 minutes, and 2, 4, 8, 12, 20 and 24 hours after the
end of the infusion. These were centrifuged and the plasma frozen
until analysed by HPLC. On subsequent cycles samples were taken
at 10mins, 1hr and 24hrs after the end of the short infusion.
Clinical information such as height, weight, age and
pre-treatment biochemistry was also recorded for each of the
patients. A population PK model was developed using the computer
program NONMEM (2). The population parameter estimates were then
used in a Bayesian Algorithm to produce posterior PK parameter
estimates for the individual, which were in turn used to predict
the epirubicin concentrations in that individual following the
next dose.
Results and Discussion
Previous studies suggest that both the inter and intra-patient
variability in PK parameter values for epirubicin is large. The
results of this study will be presented and some of the practical
problems encountered will be discussed.
1. M. Gibaldi (1991) Clinical Biopharmaceutics and
Pharmacokinetics, 4th ed, Lea and Febiger, Philadelphia
2. NONMEM User's Guide (1992), Version IV, University of
California




Next: 31
Performance Evaluation of
Up: PAGE '95: ABSTRACT LIST
Previous: 29
Modeling Interoccasion Variability
harnisch@pollux.zedat.fu-berlin.de
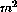 epirubicin, and 25mg prednisolone twice
daily for 5 days. 35 of these patients were randomised to receive
quinidine (250mg), as a modulator of resistance to epirubicin, or
placebo for 4 days prior to and 2 days after chemotherapy as part
of an on-going trial. Unless there was disease progression or
undue toxicity patients received 8 cycles of chemotherapy.
Epirubicin was given by a short intravenous infusion over 5-10
mins on the first cycle blood samples were taken at 0, 5, 10, 20,
40, 60 and 90 minutes, and 2, 4, 8, 12, 20 and 24 hours after the
end of the infusion. These were centrifuged and the plasma frozen
until analysed by HPLC. On subsequent cycles samples were taken
at 10mins, 1hr and 24hrs after the end of the short infusion.
Clinical information such as height, weight, age and
pre-treatment biochemistry was also recorded for each of the
patients. A population PK model was developed using the computer
program NONMEM (2). The population parameter estimates were then
used in a Bayesian Algorithm to produce posterior PK parameter
estimates for the individual, which were in turn used to predict
the epirubicin concentrations in that individual following the
next dose.
epirubicin, and 25mg prednisolone twice
daily for 5 days. 35 of these patients were randomised to receive
quinidine (250mg), as a modulator of resistance to epirubicin, or
placebo for 4 days prior to and 2 days after chemotherapy as part
of an on-going trial. Unless there was disease progression or
undue toxicity patients received 8 cycles of chemotherapy.
Epirubicin was given by a short intravenous infusion over 5-10
mins on the first cycle blood samples were taken at 0, 5, 10, 20,
40, 60 and 90 minutes, and 2, 4, 8, 12, 20 and 24 hours after the
end of the infusion. These were centrifuged and the plasma frozen
until analysed by HPLC. On subsequent cycles samples were taken
at 10mins, 1hr and 24hrs after the end of the short infusion.
Clinical information such as height, weight, age and
pre-treatment biochemistry was also recorded for each of the
patients. A population PK model was developed using the computer
program NONMEM (2). The population parameter estimates were then
used in a Bayesian Algorithm to produce posterior PK parameter
estimates for the individual, which were in turn used to predict
the epirubicin concentrations in that individual following the
next dose.