



Next: 17
Relationship Between the
Up: PAGE '95: ABSTRACT LIST
Previous: 15
Pharmacokinetic/-Dynamic Modeling of
L. Harnisch(1) ,
W. Weber(1) ,
E. Sultan(2)
Clinical Research, Hoechst AG, Frankfurt/Main, Germany(1)
Roussel Uclaf S.A., Paris, France(2)
Problem
If a drug is exclusively eliminated by a saturable process, a
steady state concentration will only be established, when the
daily dose rate (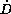 ) is less than the maximum elimination
capacity. In contrast, daily dose rates higher than the maximum
elimination capacity would lead to ever and ever increasing
plasma concentrations and in turn caused unavoidably safety
problems. Only the existence of a parallel linear elimination
would limit this increase of concentration. But of course higher
dose rates correspond to much higher steady state concentrations,
which still might cause adverse events. Single dose studies in
humans clearly demonstrated nonlinear pharmacokinetics of a new
drug. To continue its development a suggestion for a dosing rule
which achieves both safe and efficious treatment for all patients
was needed.
) is less than the maximum elimination
capacity. In contrast, daily dose rates higher than the maximum
elimination capacity would lead to ever and ever increasing
plasma concentrations and in turn caused unavoidably safety
problems. Only the existence of a parallel linear elimination
would limit this increase of concentration. But of course higher
dose rates correspond to much higher steady state concentrations,
which still might cause adverse events. Single dose studies in
humans clearly demonstrated nonlinear pharmacokinetics of a new
drug. To continue its development a suggestion for a dosing rule
which achieves both safe and efficious treatment for all patients
was needed.
Method & Materials
25 patients were dosed daily for 1 week with 50, 100, and 150
mg/day. Full kinetic profiles were drawn at day 1 and 7, while in
between only trough levels were determined. NONMEM was used for
the model building process. First a linear 2-compartment model,
subsequent a one compartment model with exclusive
Michaelis-Menten elimination and in turn with a parallel linear
elimination process were tested. The best model contained both a
non linear and a linear elimination process, and therefore all
doses tested lead to finite steady state concentrations. This
best model was used to predict sparse data of a prospectively
planned therapeutic trial. A dose of 75 mg was administered daily
in 20 patients and a total of 80 blood samples were analysed.
Results
In the population studied volume of distribution was determined
as 10 L, 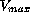 ranged from 35 mg/day to 250 mg/day and km
spread between 2 and 20 mg/L. The additional linear elimination
was characterized by a clearance of less than 1 mL/min. As a
consequence of these findings and according to the height of the
chosen dose, the treated patients split up into three groups. A
first group, defined by the condition
ranged from 35 mg/day to 250 mg/day and km
spread between 2 and 20 mg/L. The additional linear elimination
was characterized by a clearance of less than 1 mL/min. As a
consequence of these findings and according to the height of the
chosen dose, the treated patients split up into three groups. A
first group, defined by the condition 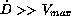 ,
establishes very high steady state concentrations and therefore
could cause toxic reactions. A second group, defined by the
alternative
,
establishes very high steady state concentrations and therefore
could cause toxic reactions. A second group, defined by the
alternative 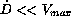 , ends up in very low steady
state concentrations, not necessarily above a therapeutic target
level. In contrast to the first and second group, the last group
with dose rates
, ends up in very low steady
state concentrations, not necessarily above a therapeutic target
level. In contrast to the first and second group, the last group
with dose rates 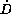 in between the limits of
in between the limits of 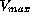 show
a nonlinear kinetic behaviour with the consequence of highly
variable and in clinical practice nearly unpredictable
steady-state concentrations.
show
a nonlinear kinetic behaviour with the consequence of highly
variable and in clinical practice nearly unpredictable
steady-state concentrations.
Therapeutic Conclusions
The splitting of the patients into three groups is the main
source of variability of the relationship between dose rate and
concentration. Less variability is expected using dose rates
either well above 250 mg/day or well below 35 mg/day.
Unfortunately both doses are not applicable for a successful
treatment, either with respect to safety or to efficacy. To
control the high variability of the pharmacokinetics of doses
between 35 and 250 mg/day some therapeutic drug monitoring might
be necessary in clinical settings.




Next: 17
Relationship Between the
Up: PAGE '95: ABSTRACT LIST
Previous: 15
Pharmacokinetic/-Dynamic Modeling of
harnisch@pollux.zedat.fu-berlin.de
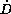 ) is less than the maximum elimination
capacity. In contrast, daily dose rates higher than the maximum
elimination capacity would lead to ever and ever increasing
plasma concentrations and in turn caused unavoidably safety
problems. Only the existence of a parallel linear elimination
would limit this increase of concentration. But of course higher
dose rates correspond to much higher steady state concentrations,
which still might cause adverse events. Single dose studies in
humans clearly demonstrated nonlinear pharmacokinetics of a new
drug. To continue its development a suggestion for a dosing rule
which achieves both safe and efficious treatment for all patients
was needed.
) is less than the maximum elimination
capacity. In contrast, daily dose rates higher than the maximum
elimination capacity would lead to ever and ever increasing
plasma concentrations and in turn caused unavoidably safety
problems. Only the existence of a parallel linear elimination
would limit this increase of concentration. But of course higher
dose rates correspond to much higher steady state concentrations,
which still might cause adverse events. Single dose studies in
humans clearly demonstrated nonlinear pharmacokinetics of a new
drug. To continue its development a suggestion for a dosing rule
which achieves both safe and efficious treatment for all patients
was needed.
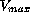 ranged from 35 mg/day to 250 mg/day and km
spread between 2 and 20 mg/L. The additional linear elimination
was characterized by a clearance of less than 1 mL/min. As a
consequence of these findings and according to the height of the
chosen dose, the treated patients split up into three groups. A
first group, defined by the condition
ranged from 35 mg/day to 250 mg/day and km
spread between 2 and 20 mg/L. The additional linear elimination
was characterized by a clearance of less than 1 mL/min. As a
consequence of these findings and according to the height of the
chosen dose, the treated patients split up into three groups. A
first group, defined by the condition 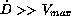 ,
establishes very high steady state concentrations and therefore
could cause toxic reactions. A second group, defined by the
alternative
,
establishes very high steady state concentrations and therefore
could cause toxic reactions. A second group, defined by the
alternative 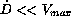 , ends up in very low steady
state concentrations, not necessarily above a therapeutic target
level. In contrast to the first and second group, the last group
with dose rates
, ends up in very low steady
state concentrations, not necessarily above a therapeutic target
level. In contrast to the first and second group, the last group
with dose rates 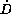 in between the limits of
in between the limits of 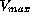 show
a nonlinear kinetic behaviour with the consequence of highly
variable and in clinical practice nearly unpredictable
steady-state concentrations.
show
a nonlinear kinetic behaviour with the consequence of highly
variable and in clinical practice nearly unpredictable
steady-state concentrations.