



Next: 10
A Prospective Evaluation
Up: PAGE '95: ABSTRACT LIST
Previous: 8
Population Distribution of
N.J. Bruce,
A.H. Thomson,
H.L. Elliott
University Department of Medicine and Therapeutics, Western
Infirmary, Glasgow, Scotland
The population pharmacokinetics of the immunosuppressant drug
tacrolimus were investigated in 172 patients, aged 18--66 years,
following liver transplantation. Tacrolimus was initially
adminstered by intravenous infusion at doses of 0.2-7mg/day then
given orally at doses of 1-20mg twice daily. Trough
concentrations were measured in whole blood from 4 to 382 days
after transplantation (median 101 days). Before analysis,
patients were randomly allocated to either the population data
set (115 patients) or the ''test'' data set (57 patients).
The population data set comprised 781 concentration measurements
and was analysed using NONMEM. Absorption was assumed to be
first-order and the absorption rate constant was fixed at
0.5h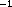 . Elimination was satisfactorily described using a
monoexponential decline. The following clinical factors were
tested for their influence on clearance (CL), volume of
distribution (V), and bioavailability (F): sex; age; height;
weight; body mass index; body surface area; day; indices of
hepatic function (concentrations of protein, albumin, bilirubin,
alkaline phosphatase (ALKP), alanine amino transferase (ALT),
aspartate amino tranferase (AST) and glutamyl amino transferase
(GGT); indices of renal function (creatinine concentration,
estimated creatinine clearance); and haematocrit. The influence
of these covariates was investigated using linear, non- linear
and step models. Model comparisons were based on the change in
objective function value (chi-squared test, p<0.01
significant), residual plots and the standard errors of the
parameter estimates.
. Elimination was satisfactorily described using a
monoexponential decline. The following clinical factors were
tested for their influence on clearance (CL), volume of
distribution (V), and bioavailability (F): sex; age; height;
weight; body mass index; body surface area; day; indices of
hepatic function (concentrations of protein, albumin, bilirubin,
alkaline phosphatase (ALKP), alanine amino transferase (ALT),
aspartate amino tranferase (AST) and glutamyl amino transferase
(GGT); indices of renal function (creatinine concentration,
estimated creatinine clearance); and haematocrit. The influence
of these covariates was investigated using linear, non- linear
and step models. Model comparisons were based on the change in
objective function value (chi-squared test, p<0.01
significant), residual plots and the standard errors of the
parameter estimates.
Clearance was reduced in patients with evidence of liver
impairment, as indicated by AST concentrations greater than
85 uL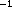 , ALT concentrations greater than 850 uL
, ALT concentrations greater than 850 uL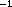 , or
bilirubin concentrations greater than 245
, or
bilirubin concentrations greater than 245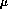 molL
molL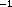 . The
best model included all three covariates. No relationships were
identified between any of the covariates and volume of
distribution. Bioavailability was linearly related to time (day)
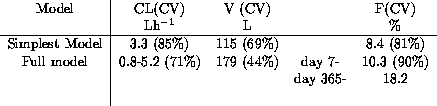
post-transplant. The range of parameter estimates determined
using the full model and the simplest model are summarised in
Table 1.
. The
best model included all three covariates. No relationships were
identified between any of the covariates and volume of
distribution. Bioavailability was linearly related to time (day)
post-transplant. The range of parameter estimates determined
using the full model and the simplest model are summarised in
Table 1.
Table 1:
These population derived results were evaluated using the
''test'' data set which comprised 378 concentrations. The
predicted distribution of each concentration (mean 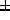 SD) was
determined using the parameter estimates obtained in the
population analysis. For each individual the percentage of
measured concentrations that lay within this distribution was
calculated. Eighty- three percent of the measured concentrations
for the group as a whole were within their predicted
distributions but there was a small bias in the mean predicted
concentration (2.3
SD) was
determined using the parameter estimates obtained in the
population analysis. For each individual the percentage of
measured concentrations that lay within this distribution was
calculated. Eighty- three percent of the measured concentrations
for the group as a whole were within their predicted
distributions but there was a small bias in the mean predicted
concentration (2.3 8.6ng/ml).
8.6ng/ml).
This analysis has identified significant relationships in liver
transplant patients between the pharmacokinetics of tacrolimus
and both indices of hapatic function and time post transplant.




Next: 10
A Prospective Evaluation
Up: PAGE '95: ABSTRACT LIST
Previous: 8
Population Distribution of
harnisch@pollux.zedat.fu-berlin.de
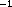 . Elimination was satisfactorily described using a
monoexponential decline. The following clinical factors were
tested for their influence on clearance (CL), volume of
distribution (V), and bioavailability (F): sex; age; height;
weight; body mass index; body surface area; day; indices of
hepatic function (concentrations of protein, albumin, bilirubin,
alkaline phosphatase (ALKP), alanine amino transferase (ALT),
aspartate amino tranferase (AST) and glutamyl amino transferase
(GGT); indices of renal function (creatinine concentration,
estimated creatinine clearance); and haematocrit. The influence
of these covariates was investigated using linear, non- linear
and step models. Model comparisons were based on the change in
objective function value (chi-squared test, p<0.01
significant), residual plots and the standard errors of the
parameter estimates.
. Elimination was satisfactorily described using a
monoexponential decline. The following clinical factors were
tested for their influence on clearance (CL), volume of
distribution (V), and bioavailability (F): sex; age; height;
weight; body mass index; body surface area; day; indices of
hepatic function (concentrations of protein, albumin, bilirubin,
alkaline phosphatase (ALKP), alanine amino transferase (ALT),
aspartate amino tranferase (AST) and glutamyl amino transferase
(GGT); indices of renal function (creatinine concentration,
estimated creatinine clearance); and haematocrit. The influence
of these covariates was investigated using linear, non- linear
and step models. Model comparisons were based on the change in
objective function value (chi-squared test, p<0.01
significant), residual plots and the standard errors of the
parameter estimates.
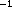 , ALT concentrations greater than 850 uL
, ALT concentrations greater than 850 uL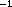 , or
bilirubin concentrations greater than 245
, or
bilirubin concentrations greater than 245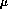 molL
molL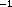 . The
best model included all three covariates. No relationships were
identified between any of the covariates and volume of
distribution. Bioavailability was linearly related to time (day)
post-transplant. The range of parameter estimates determined
using the full model and the simplest model are summarised in
Table 1.
. The
best model included all three covariates. No relationships were
identified between any of the covariates and volume of
distribution. Bioavailability was linearly related to time (day)
post-transplant. The range of parameter estimates determined
using the full model and the simplest model are summarised in
Table 1.

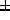 SD) was
determined using the parameter estimates obtained in the
population analysis. For each individual the percentage of
measured concentrations that lay within this distribution was
calculated. Eighty- three percent of the measured concentrations
for the group as a whole were within their predicted
distributions but there was a small bias in the mean predicted
concentration (2.3
SD) was
determined using the parameter estimates obtained in the
population analysis. For each individual the percentage of
measured concentrations that lay within this distribution was
calculated. Eighty- three percent of the measured concentrations
for the group as a whole were within their predicted
distributions but there was a small bias in the mean predicted
concentration (2.3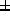 8.6ng/ml).
8.6ng/ml).